Trethera and UCLA Publish Data Demonstrating TRE-515 Ability to Control Immune Cell Activation That Causes Multiple Sclerosis Symptoms in Mice
/EIN News/ -- LOS ANGELES, Feb. 25, 2025 (GLOBE NEWSWIRE) -- Trethera Corporation (“Trethera”), a clinical stage biopharmaceutical company committed to targeting cancer and autoimmune diseases through first-in-class drugs, announced research results in the peer-reviewed journal Immunology demonstrating that TRE-515 selectively inhibits autoreactive, aberrantly activated lymphocytes to improve multiple sclerosis (MS) symptoms in mouse models. TRE-515, a clinical stage drug developed by Trethera, targets the enzyme deoxycytidine kinase (dCK) in the deoxyribonucleoside salvage pathway.
When T and B cells become activated to attack the body in an autoimmune disease such as MS, these cells rapidly divide. By inhibiting rapid division, there are not enough cells to cause nerve damage. In collaborative work, Trethera and the Peter Clark Lab at UCLA have shown that when autoreactive T and B cells divide, they activate the TRE-515 target dCK. In those abnormally activated cells, dCK is directly required for rapid cell division. The researchers have shown in a carefully controlled cell culture system that without dCK, the disease-causing T and B cells were unable to make enough of the required nucleotide building blocks such as dCTP for the DNA replication that occurs during cell division. Targeting dCK with TRE-515 slowed the rate of T and B cell division, leading to fewer cells.
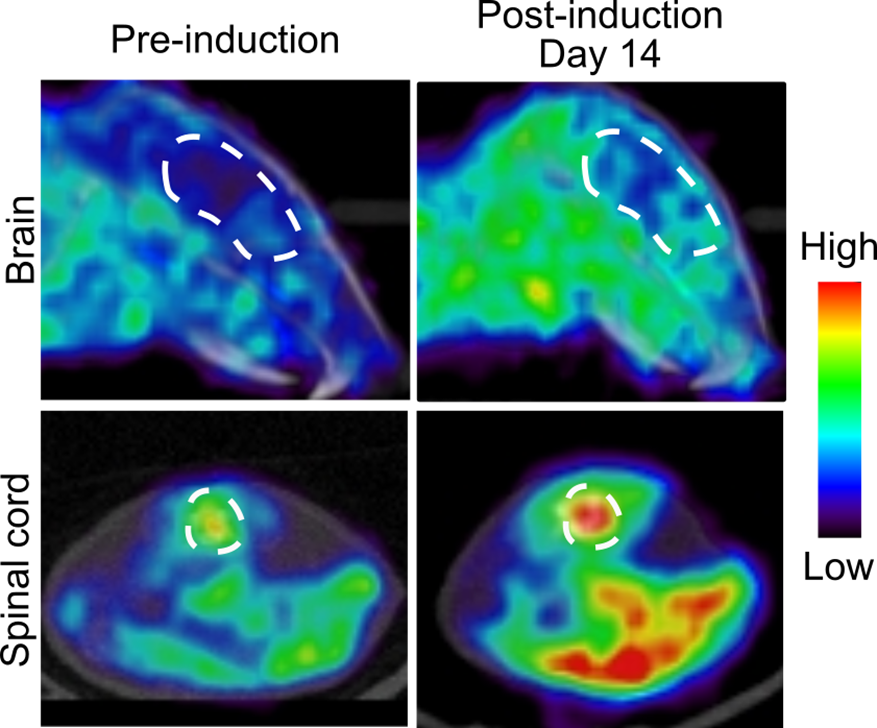
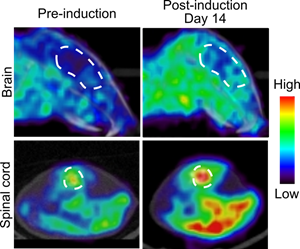
Figure 1: PET scans showing mouse brain and spinal cord dCK activity before (left) and after (right) disease induction. The enzyme dCK becomes upregulated during MS disease.
These results were translated into the proteolipid protein (PLP139-151) mouse models of MS where the researchers found dCK was activated during disease and pharmacological inhibition of dCK with TRE-515 led to fewer autoreactive T and B cells and less disease. Unlike many available MS therapies, TRE-515 accomplishes this without affecting the levels of other immune cell populations such as naïve T and B cells or innate immune cells. These results suggest that dCK represents a potential new target for treating patients and modulating symptoms in MS and other demyelinating diseases. This study builds on findings published in the 2023 Immunology cover article (PMID: 35986643).
"These findings strongly support dCK as a breakthrough target for treating T-cell and B-cell mediated demyelinating diseases such as MS, optic neuritis, and ADEM. The observation that TRE-515 achieves efficacy without disrupting other immune cell populations—such as naïve T and B cells or innate immune cells—is particularly impressive. This growing body of evidence positions dCK as a promising new frontier for treating MS and related conditions," said Dr. Larry Steinman, Trethera Scientific Advisory Board member. Dr. Steinman is a noted immunologist and neurologist, a professor of pediatrics at Stanford University, and a member of the National Academy of Science who was not directly involved in the research.
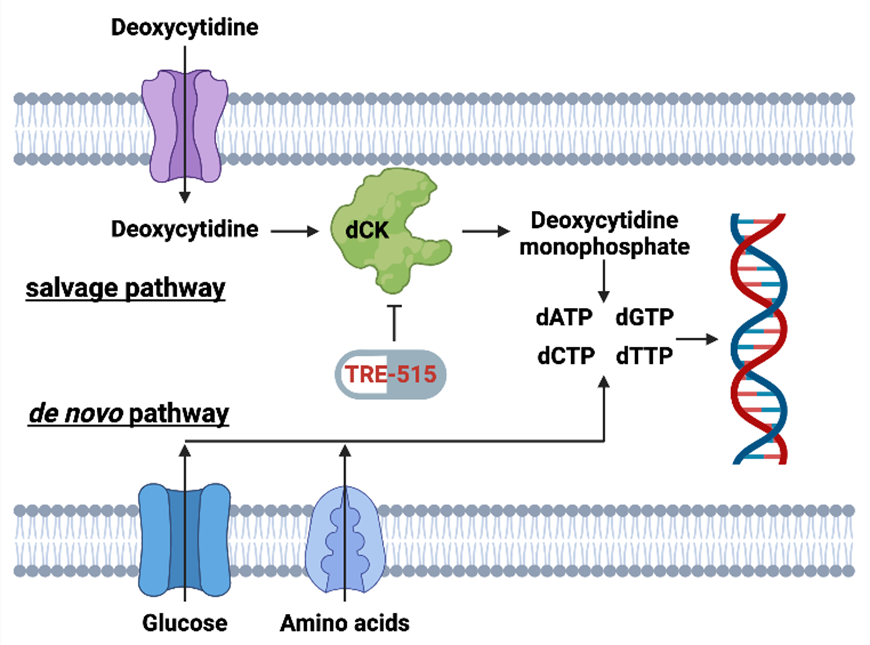
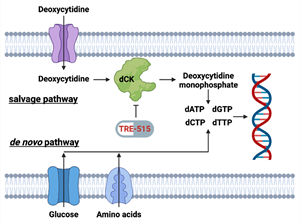
Figure 2: Biochemical pathways for the supply of deoxycytidine triphosphate pools. The salvage pathway becomes upregulated during MS disease. FDA approved MS drugs blocking the de novo pathway include Aubagio (teriflunomide) and Mavenclad (cladribine).
Reference: Salas et al. (2025), Blocking Deoxycytidine Kinase in Activated Lymphocytes Depletes Deoxycytidine Triphosphate Pools and Alters Cell Cycle Kinetics to Yield Less Disease in a Mouse Multiple Sclerosis Model. Immunology: Volume 174, Issue 2, February 2025 (https://doi.org/10.1111/imm.13885).
About Trethera
Trethera is a clinical stage, privately held, biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera's innovative approach to targeting nucleotide metabolism led to the development of TRE-515, an orally administered capsule twice designated by the FDA as an Orphan Drug. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the nucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases might also respond to TRE-515 treatment. Trethera is developing TRE-515 for use as a monotherapy or in combination to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
For more information, please visit us at trethera.com or e-mail Investor Relations at ir@trethera.com.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are “forward-looking statements,” which may often, but not always, be identified by the use of such words as "may," "might," "will," "will likely result," "would," "should," "estimate," "plan," "project," "forecast," "intend," "expect," "anticipate," "believe," "seek," "continue," "target" or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera’s control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/de328b18-3a17-48af-8428-efe2b0f1f9ed
https://www.globenewswire.com/NewsRoom/AttachmentNg/d7fb9306-32a1-4845-96ca-921089b8e7a0

Figure 1
PET scans showing mouse brain and spinal cord dCK activity before (left) and after (right) disease induction. The enzyme dCK becomes upregulated during MS disease.
Figure 2
Biochemical pathways for the supply of deoxycytidine triphosphate pools. The salvage pathway becomes upregulated during MS disease. FDA approved MS drugs blocking the de novo pathway include Aubagio (teriflunomide) and Mavenclad (cladribine).
Distribution channels: Media, Advertising & PR, Technology ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release